Developing medical technologies has traditionally been slow and costly. Taking a new device from concept to market can take years, as technology development must align with regulatory requirements and cost constraints. VTT’s medical device pilot line helps companies turn ideas into practical prototypes without heavy upfront investments.
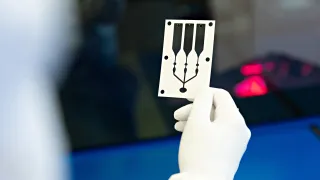
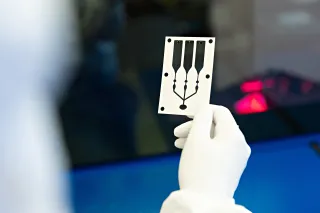
At VTT’s medical device pilot line in Oulu, health and wellbeing technology companies can develop and test their device concepts quickly and flexibly. The pilot line utilizes VTT’s printed and flexible electronics technologies, which enable the manufacturing of lightweight, skin-attachable sensors and other measurement solutions that monitor the body’s functions unobtrusively and comfortably.
“Our goal is to bring measurement closer to everyday life. When a sensor is lightweight and unobtrusive, it can be used at home or on the move while still delivering accurate, reliable data”, says Markus Tuomikoski, Research Team Leader at VTT.
Skin-attachable wireless sensors can be used in preventive cardiovascular monitoring, metabolic syndrome tracking, early cancer detection and rapid diagnostics.
A flexible partner for both startups and large enterprises
Customers of the pilot line range from global medical technology corporations to early-stage startups. For startups, the pilot line offers a way to validate their idea without major initial investments and to produce rapid prototypes that help convince investors and development partners.
“Customers don’t need to come to us with a perfect plan. A clear need or concept is enough. We help building a functioning technical solution around it”, Tuomikoski explains.
The collaboration proceeds step by step: VTT signs a non-disclosure agreement, prepares a proposal tailored to the customer’s needs and executes the project either as a comprehensive development effort or as individual process steps. The results and intellectual property remain with the customer.
World-class infrastructure and expertise
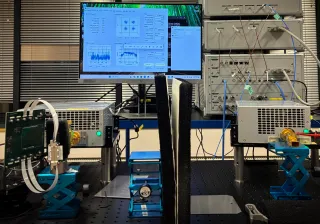
The medical technology pilot line combines PrintoCent’s printed electronics pilot factory with ISO7-class cleanrooms, enabling the manufacturing of both sheet-based and roll-to-roll structures. This makes the facility exceptionally versatile, and it supports both medical device prototypes and wellbeing technology development.
Key infrastructure includes:
- State-of-the-art ISO7 cleanroom
- Flatbed automatic screen printer (sheets up to 500 mm x 500 mm)
- UV and IR belt oven
- Flatbed Computer-to-Screen (CtS) screen exposure system,
- Automatic component assembly line for large area flexible PCBs
- High-capacity 3D X-ray Microscopy, surface profilometers and material characterization capabilities
- Picosecond UV Laser for cutting, structuring, and drilling of flexible PCBs
- High precision press flat die cutter
- Advanced Sub-Micron Bonder for photonics packaging
- Photonics packaging capabilities
- Advanced testing and reliability capabilities for flexible electronics and photonics
- High-precision dispenser for bioreagents
Bridging the gap between research and industrial production
In medical technology development, the transition from research to commercial mass production often requires such considerable time and investment that it is commonly called the “valley of death”. VTT’s expertise and pilot-scale manufacturing capacity are designed to help companies overcome this critical phase.
“Our job is to develop the manufacturing process and transfer it to the customer. Later, the company can choose a contract manufacturer or build its own production. When needed, we also help assess required investments, production volumes and suitable partners”, says Antti Kemppainen, Solution Sales Lead, Sensing Solutions, at VTT.
VTT’s extensive international network and long experience in combining electronics, biotechnology and data ensure that customers receive not only technical expertise but also connections to the right industry partners.
A faster path to market
Some projects at the pilot line produce their first prototypes in just a few weeks. Larger projects typically take from six months to a year.
“We have the capabilities from rapid experimentation towards long-term technology development. High quality guides all our work. Testing, characterization and reliability assessments are always carried out in-house”, Kemppainen notes.
The pilot line offers companies a ready-made environment where ideas can be tested, developed and refined before major production decisions. The market entry of a new medical device is significantly accelerated when technology, reliability and user experience can be validated early.
“In the end, it’s about ensuring that good ideas don’t stay in a drawer. We help turn them into prototypes for user testing and support the path to market through further development and technology transfer”, Kemppainen says.
Meet our team

Markus Tuomikoski works at VTT as Head of Printed Electronics Processing Team. He is responsible for multidisciplinary research and development projects, where new technologies are transformed from ideas into functional prototypes. His expertise encompasses the entire development pathway from conceptualisation and material selection to manufacturing design, testing and validation. Markus has extensive experience leading both national and international R&D projects, as well as a broad network of collaborators in industry and academia.
In his role, Markus combines technology development, people management and practical impact. At the heart of his work is how VTT’s state-of-the-art research infrastructure and expert teams help to develop new technologies to meet tangible societal and industrial needs. He notes that healthcare in particular requires innovative solutions to ensure the system continues to function in an ageing society.

Antti Kemppainen is a Sales Manager at VTT, where he focuses on developing and applying flexible electronics and photonic measurement solutions. He has 25 years’ experience in developing printed electronics and advancing their commercial opportunities. Antti has participated in numerous projects where new technologies have been advanced toward functional prototypes and further development.
Antti is internationally recognised within the printed electronics community as a pioneer and an active contributor in discussions within the field. He is motivated by the opportunity to work with new and highly specific technologies as well as close collaboration with VTT’s experts and the global technology network.
For Antti, the significance of his work lies in how research supports company growth and the commercialisation of innovations. In particular, the success of growth companies depends on being able to take the first steps in development quickly and in the right environment.